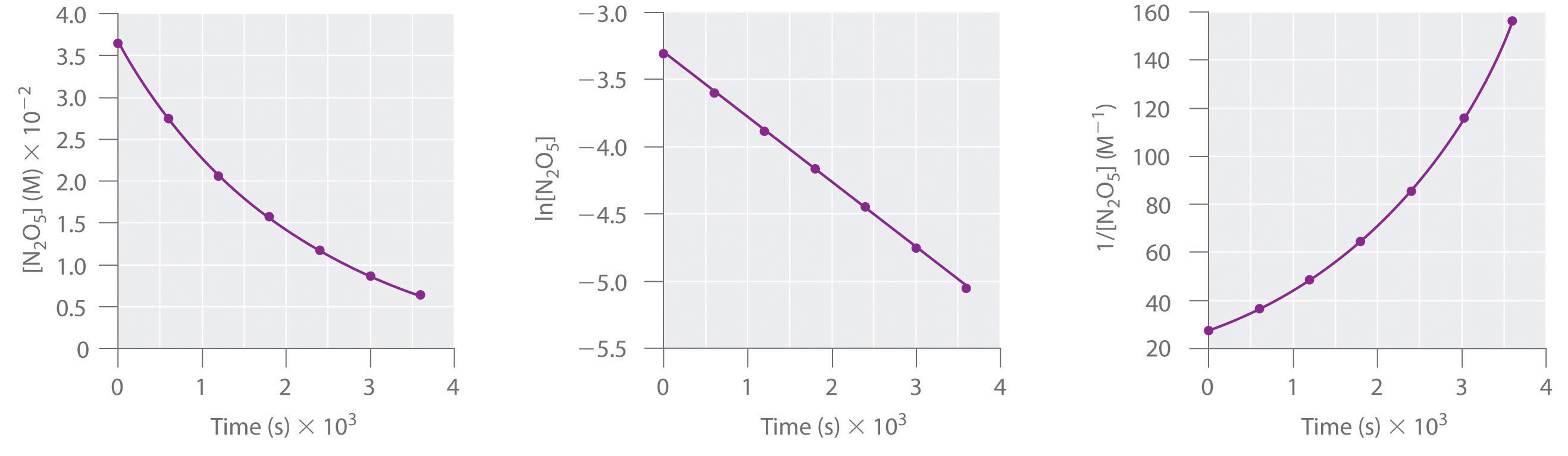
The Rate Constant For 1St Order Reaction Is 69.3 . T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant?
from chem.libretexts.org
ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant?
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and
The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From studylib.net
A first order reaction has a rate constant of 1 The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From chemguru.sg
Rate Equation and Order of Reaction The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. In other words, if the. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
First Order Reactions Chemistry Class 12 IIT JEE Main + Advanced The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.brainkart.com
Rate equation for first order reactions The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.meritnation.com
Rate constant of a first order reaction is 2 3*10^4 s^ calculate the The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From dxotqwilz.blob.core.windows.net
Formula Of Rate Constant For First Order Reaction at John Kingston blog The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. In other words, if the. T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders The Rate Constant For 1St Order Reaction Is 69.3 ln[a] = − kt + ln[a]o. T 1 / 2 = 69.3 minutes. In other words, if the. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Units of rate constant calculate rate constant units units of first The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. In other words, if the. ln[a] = − kt + ln[a]o. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
The rate constant of a first order reaction is `3 xx 10^(6)" per sec The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. T 1 / 2 = ln 2 k. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. In other words, if the. The Rate Constant For 1St Order Reaction Is 69.3.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and The Rate Constant For 1St Order Reaction Is 69.3 In other words, if the. T 1 / 2 = ln 2 k. T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? ln[a] = − kt + ln[a]o. The Rate Constant For 1St Order Reaction Is 69.3.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial The Rate Constant For 1St Order Reaction Is 69.3 T 1 / 2 = 69.3 minutes. If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.
From askfilo.com
Units of rate constant for a first order reaction. Filo The Rate Constant For 1St Order Reaction Is 69.3 If the half life period for a first order reaction is 69.3 seconds, what is the value of its rate constant? In other words, if the. T 1 / 2 = 69.3 minutes. ln[a] = − kt + ln[a]o. T 1 / 2 = ln 2 k. The Rate Constant For 1St Order Reaction Is 69.3.